This guide explores the evolving landscape of cancer diagnostics through the lens of liquid biopsy and tissue biopsy. It highlights their distinct advantages, challenges, and integration prospects, offering insights into the future of personalized cancer treatment and patient care.
Introduction:
Cancer diagnosis has traditionally relied on tissue biopsies, where a small sample of the tumor or affected tissue is extracted for analysis. However, recent advancements in medical technology have introduced liquid biopsy as a promising alternative. This comprehensive guide delves into the intricate differences between liquid biopsy and tissue biopsy, their respective roles in cancer diagnostics, and the potential impact they hold for the future of cancer treatment and patient care.
Understanding Tissue Biopsy:
Tissue biopsy is a well-established method in cancer diagnosis, involving the surgical removal of a sample of the tumor or affected tissue. This sample is then examined under a microscope to determine the tumor's histological type, grade, genetic mutations, and other characteristics. Tissue biopsy provides crucial information that guides treatment decisions, such as choosing the most effective therapies and predicting the tumor's behavior.
One of the primary advantages of tissue biopsy is its ability to provide detailed insights into the tumor's structure and composition. Pathologists can analyze the tissue sample to identify specific biomarkers, assess the tumor's aggressiveness, and determine its responsiveness to various treatments. Tissue biopsy remains a cornerstone in cancer diagnostics, particularly for solid tumors where tissue samples are readily accessible.
However, tissue biopsy does have limitations. It is an invasive procedure that requires surgical intervention, which can be associated with risks such as bleeding, infection, and discomfort for the patient. Additionally, obtaining tissue samples may not always be feasible, especially for tumors located in challenging anatomical sites or in patients with medical conditions that make surgery difficult.
Exploring Liquid Biopsy:
Liquid biopsy represents a paradigm shift in cancer diagnostics, offering a less invasive and more accessible approach to detecting and monitoring cancer. Instead of extracting tissue samples, liquid biopsy analyzes biomarkers present in bodily fluids such as blood, urine, or cerebrospinal fluid. These biomarkers include circulating tumor cells (CTCs), cell-free DNA (cfDNA), microRNAs, proteins, and exosomes shed by tumors into the bloodstream or other bodily fluids.
The primary advantage of liquid biopsy is its minimally invasive nature. A simple blood draw or urine sample can provide valuable insights into the tumor's genetic makeup, mutations, and molecular characteristics. Liquid biopsy is particularly beneficial for detecting early-stage cancers, monitoring treatment response, detecting minimal residual disease (MRD), and identifying potential drug resistance mutations.
Liquid biopsy holds promise for several key applications in cancer diagnostics and management:
Early Detection: Liquid biopsy can detect cancer at an early stage by identifying circulating tumor cells or tumor-derived DNA in the bloodstream before tumors are detectable by imaging or symptomatic.
Monitoring Treatment Response: Liquid biopsy allows for real-time monitoring of treatment response and disease progression. Changes in biomarker levels can indicate treatment efficacy or the emergence of resistance, guiding adjustments to therapy.
Minimal Residual Disease (MRD) Monitoring: After initial treatment, liquid biopsy can detect residual cancer cells that may indicate a risk of disease recurrence. This information helps in implementing targeted interventions to prevent disease relapse.
Biomarker Discovery: Liquid biopsy facilitates the discovery of novel biomarkers associated with cancer development, progression, and response to therapy. These biomarkers can inform the development of targeted therapies and personalized treatment plans.
Non-invasive Surveillance: Liquid biopsy enables non-invasive surveillance of high-risk individuals, such as those with a family history of cancer or individuals with genetic predispositions to certain cancers.
Key Differences between Liquid Biopsy and Tissue Biopsy:
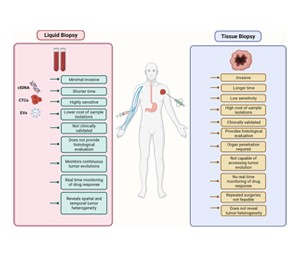
Invasiveness: Tissue biopsy is an invasive procedure that requires a surgical intervention to obtain a tissue sample, while liquid biopsy is minimally invasive, typically involving a simple blood draw or urine sample.
Timing and Frequency: Tissue biopsy is often performed at specific time points, such as during initial diagnosis or after treatment, while liquid biopsy can be performed more frequently to monitor treatment response and disease progression.
Sensitivity and Specificity: Liquid biopsy can detect genetic mutations and biomarkers associated with cancer at very low concentrations, making it potentially more sensitive than tissue biopsy in certain contexts. However, tissue biopsy provides detailed histological and morphological information that may not be accessible through liquid biopsy alone.
Accessibility: Liquid biopsy is more accessible and convenient for patients, especially those who may not be candidates for surgical procedures or who require frequent monitoring during treatment.
Here's a concise table highlighting the key differences between liquid biopsy and tissue biopsy:
|
Aspect |
Liquid Biopsy |
Tissue Biopsy |
|
Invasiveness |
Minimally invasive, often a blood draw or urine sample |
Invasive, requires surgical removal of tissue |
|
Timing/Frequency |
Can be performed more frequently for monitoring | Typically performed at specific time points |
| Sensitivity | Can detect genetic mutations at low concentrations |
Provides detailed histological and morphological information |
|
Accessibility |
More accessible, especially for high-risk patients | Limited accessibility for certain tumor locations |
|
Monitoring |
Real-time monitoring of treatment response |
Limited to specific time points during treatment |
This table summarizes the primary differences between liquid biopsy and tissue biopsy in terms of invasiveness, timing/frequency of performance, sensitivity, accessibility, and monitoring capabilities.
The Future of Cancer Diagnostics:
Both liquid biopsy and tissue biopsy play integral roles in cancer diagnostics, and their integration offers a comprehensive approach to understanding and managing cancer. The future of cancer diagnostics lies in leveraging the strengths of both methods to optimize patient care and treatment outcomes.
Liquid biopsy holds immense potential for advancing personalized medicine in oncology. As technology continues to evolve, liquid biopsy techniques are becoming increasingly sensitive, allowing for the detection of rare genetic mutations and the characterization of tumor heterogeneity. These advancements enable oncologists to tailor treatment plans based on the unique molecular profile of each patient's cancer, maximizing therapeutic efficacy and minimizing side effects.
Furthermore, liquid biopsy has the potential to revolutionize cancer screening programs by offering non-invasive methods for early detection and monitoring of high-risk individuals. This proactive approach can lead to earlier intervention, improved survival rates, and reduced healthcare costs associated with late-stage cancer treatment.
On the other hand, tissue biopsy remains indispensable for certain aspects of cancer diagnosis and management. Histological analysis of tissue samples provides critical information about tumor morphology, grade, invasion depth, and lymph node involvement. This information guides surgical planning, determines tumor staging, and informs prognostic assessments.
The integration of liquid biopsy and tissue biopsy in clinical practice represents a comprehensive diagnostic approach termed "multimodal biopsy." Multimodal biopsy combines the strengths of both methods to provide a holistic view of the tumor, including its genetic profile, histological features, and microenvironment interactions. By integrating molecular and histopathological data, oncologists can make more informed decisions regarding treatment selection, patient stratification for clinical trials, and therapeutic monitoring.
Challenges and Considerations:
Despite the promising advancements in liquid biopsy technology, several challenges and considerations remain:
a. Standardization: Standardization of liquid biopsy protocols, sample collection methods, and analytical techniques is essential to ensure reproducibility and reliability of results across different laboratories and platforms.
b. Sensitivity and Specificity: Improving the sensitivity and specificity of liquid biopsy assays is critical for accurately detecting minimal residual disease, rare mutations, and early-stage cancers.
c. Cost-Effectiveness: Cost-effectiveness analyses are needed to evaluate the economic impact of integrating liquid biopsy into routine clinical practice, including considerations of reimbursement policies and healthcare resource utilization.
d. Clinical Validation: Large-scale clinical studies and validation trials are necessary to demonstrate the clinical utility, accuracy, and prognostic value of liquid biopsy in different cancer types and stages.
e. Regulatory Approval: Regulatory approval and validation of liquid biopsy assays by regulatory agencies, such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA), are crucial for widespread adoption and clinical implementation.
Conclusion:
Liquid biopsy and tissue biopsy represent complementary approaches to cancer diagnostics, each offering unique advantages and insights into the nature of cancer. While tissue biopsy remains essential for histological analysis and tumor characterization, liquid biopsy holds promise for non-invasive early detection, treatment monitoring, and personalized medicine in oncology.
The future of cancer diagnostics lies in harnessing the synergies between liquid biopsy and tissue biopsy to develop multimodal diagnostic strategies that integrate molecular, histopathological, and imaging data. The integrative approach enables oncologists to tailor treatment plans based on a comprehensive understanding of the tumor's molecular profile, histological characteristics, and imaging findings. By combining information from liquid biopsy, tissue biopsy, imaging modalities such as MRI, CT scans, and PET scans, as well as other diagnostic tests, oncologists can make more precise and personalized treatment decisions for each patient. This personalized approach maximizes therapeutic efficacy, minimizes potential side effects, and improves patient outcomes in the complex landscape of cancer care. Additionally, the integration of multimodal diagnostic strategies facilitates patient stratification for targeted therapies, immunotherapies, and participation in clinical trials, leading to advancements in precision oncology and ultimately, better quality of life for cancer patients.